

In 1852, Henry Fox Talbot discovered that exposure to ultraviolet light in the presence of potassium dichromate hardened organic colloids such as gelatin and gum arabic, making them less soluble. In 1839, Mungo Ponton discovered that paper treated with a solution of potassium dichromate was visibly tanned by exposure to sunlight, the discoloration remaining after the potassium dichromate had been rinsed out. This usage commonly causes contact dermatitis in construction workers. It is used as an ingredient in cement in which it retards the setting of the mixture and improves its density and texture. Because of safety concerns associated with hexavalent chromium, this practice has been largely discontinued. Like other chromium(VI) compounds ( chromium trioxide, sodium dichromate), potassium dichromate has been used to prepare " chromic acid" for cleaning glassware and etching materials. The main use is as a precursor to potassium chrome alum, used in leather tanning. Potassium dichromate has few major applications, as the sodium salt is dominant industrially. On heating with concentrated acid, oxygen is evolved:Ģ K 2Cr 2O 7 + 8 H 2SO 4 → 2 K 2SO 4 + 2 Cr 2(SO 4) 3 + 8 H 2O + 3 O 2 Uses Treatment with cold sulfuric acid gives red crystals of chromic anhydride (chromium trioxide, CrO 3): For example, potassium chromate is produced industrially using potash: When an alkali is added to an orange-red solution containing dichromate ions, a yellow solution is obtained due to the formation of chromate ions ( CrO 2− 4). When heated strongly, it decomposes with the evolution of oxygen.Ĥ K 2Cr 2O 7 → 4 K 2CrO 4 + 2 Cr 2O 3 + 3 O 2 A ketone will show no such change because it cannot be oxidized further, and so the solution will remain orange. This color change arises because the aldehyde can be oxidized to the corresponding carboxylic acid. Aldehydes reduce dichromate from the +6 to the +3 oxidation state, changing color from orange to green. In an aqueous solution the color change exhibited can be used to test for distinguishing aldehydes from ketones. For example, menthone may be prepared by oxidation of menthol with acidified dichromate. Secondary alcohols are converted into ketones. In contrast, potassium permanganate tends to give carboxylic acids as the sole products. It converts primary alcohols into aldehydes and, under more forcing conditions, into carboxylic acids. Potassium dichromate is an oxidising agent in organic chemistry, and is milder than potassium permanganate. It is soluble in water and in the dissolution process it ionizes: Alternatively, it can be also obtained from potassium chromate by roasting chromite ore with potassium hydroxide. It is also used as an analytical reagent, a mordant, and a raw material for making green inks, green paints, ceramic glazes, and other chromium salts, such as chromium trioxide.Potassium dichromate is usually prepared by the reaction of potassium chloride on sodium dichromate.
Soluble in water, slightly soluble in ethanol.Īfter reacting chromium hydroxide with sulfuric acid, it is obtained by natural evaporation and crystallization.Ĭhromium sulfate is widely used as mordant for tanning or textile, and in the preparation of chrome alum. A relative density of 1.70(22/4 °c), with loss of 12 molecules of crystal water at 100 °c.
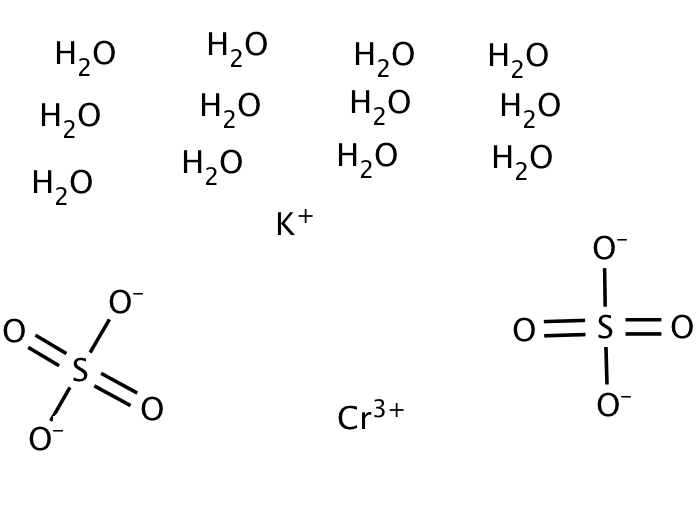
18H2O = 716.45, blue-purple cubic crystal.The relative density of 1.87(17/4 deg C), heat to 100 deg C lost very daughter crystal water. Chromium sulfate hydrate often exists in the form of 3, which appears purple in appearance, and the aqueous solution turns green by heating.

Chromium sulfate is an inorganic compound with the chemical formula Cr2(SO4)3.


 0 kommentar(er)
0 kommentar(er)
